
NABL Accreditation
NABL Accreditation Accreditation Accreditation

NABL Accreditation
National Accreditation Board for Testing and Calibration Laboratories (NABL) provides an internationally accepted certification. This certification is recognized around the world and used to prove calibration accuracy, stability, and traceability.
National Accreditation Board for Testing and Calibration Laboratories (NABL) is an internationally accredited body that is based in India. NABL was established by the Bureau of Indian Standards (BIS) to help ensure the technical credibility, independence and credibility of testing laboratories used by industry, government and other organizations.
NABL is one of the constituent boards of Quality Council of India (QCI), an autonomous body under Department for Promotion of Industry & Internal Trade (DPIIT), Ministry of Commerce and Industry, Government of India.
NABL Vision:
To be the world’s leading accreditation body and to enhance stakeholders’ confidence in its services.
NABL Mission:
To strengthen the accreditation system accepted across the globe by providing high quality, value driven services, fostering APAC/ILAC MRA, empanelling competent assessors, creating awareness among the stake holders, initiating new programs supporting accreditation activities and pursuing organisational excellence.
Why Laboratory Accreditation?
International Recognition:With an objective to ensure the acceptance of test/ calibration results issued by the accredited conformity assessment bodies (CAB) across the borders, NABL maintains linkages with the international bodies-
- International Laboratory Accreditation Co-operation (ILAC) and
- Asia Pacific Accreditation Co-operation (APAC).
NABL is a full member of ILAC and APAC and regularly takes part in their meetings.
NABL is Mutual Recognition Arrangement (MRA) signatory to ILAC as well as APAC for the accreditation programs – Testing and Calibration (ISO/IEC 17025), Medical (ISO 15189), Proficiency Testing Providers (PTP) (ISO/IEC 17043) and Reference material producers (RMP) (ISO 17034).
Accreditation Process:
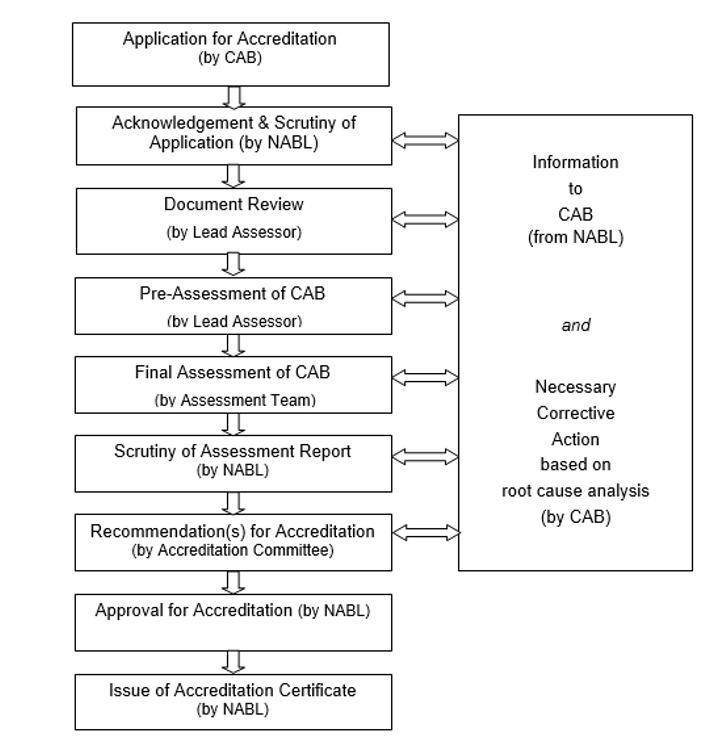
NABL grants accreditation to Testing Laboratories in accordance with ISO/ IEC 17025:
“General Requirements for the Competence of Testing and Calibration Laboratories”. The accreditation services to Testing Laboratories is currently given in the following disciplines:
- Chemical
- Biological
- Mechanical
- Electrical
- Electronics
- Fluid Flow
- Forensic
- Non-Destructive (NDT)
- Photometry
- Radiological
- Diagnostic Radiology QA Testing
- Software & IT System
NABL grants accreditation to Calibration Laboratories in accordance with ISO/ IEC 17025:
General Requirements for the Competence of Testing and Calibration Laboratories. The accreditation services to Calibration Laboratories is currently given in the following disciplines:
- Mechanical
- Electro Technical
- Fluid Flow
- Thermal
- Optical
- Medical Devices
- Radiological
NABL grants accreditation to medical testing laboratories in accordance with ISO 15189:
“Medical laboratories- requirements for quality and competence” The accreditation services to Medical Laboratories is currently given in the following disciplines:
- Clinical Biochemistry
- Clinical Pathology
- Haematology
- Microbiology & Infectious disease serology
- Histopathology
- Cytopathology
- Flow Cytometry
- Cytogenetics
- Molecular Testing
NABL grants accreditation to Proficiency Testing Providers (PTP) in accordance with ISO/ IEC 17043:
“Conformity assessment – General requirements for Proficiency Testing”
The accreditation services to Proficiency Testing Providers (PTP) is currently given in the following disciplines:
- Testing
- Calibration
- Medical
- Inspection
NABL grants accreditation to Reference Material Producers (RMP) in accordance with ISO 17034:
“General requirements for the competence of Reference Material Producers”
The accreditation services to Reference Material Producers
(RMP) is currently given in the following categories:
- Chemical Composition
- Biological & Clinical Properties
- Physical Properties
- Engineering Properties
- Miscellaneous Properties